How To Find Atomic Weight Of An Element
Chapter 2. Atoms, Molecules, and Ions
two.iii Diminutive Structure and Symbolism
Learning Objectives
By the end of this department, you will be able to:
- Write and translate symbols that describe the atomic number, mass number, and accuse of an cantlet or ion
- Define the atomic mass unit and boilerplate atomic mass
- Calculate average atomic mass and isotopic abundance
The evolution of modern atomic theory revealed much well-nigh the inner structure of atoms. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of infinite containing negatively charged electrons. The nucleus contains the majority of an atom's mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom'south book. The diameter of an cantlet is on the lodge of ten−ten yard, whereas the diameter of the nucleus is roughly ten−15 grand—nigh 100,000 times smaller. For a perspective about their relative sizes, consider this: If the nucleus were the size of a huckleberry, the atom would be about the size of a football stadium (Figure one).

Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For case, a carbon atom weighs less than 2 × 10−23 m, and an electron has a charge of less than 2 × x−19 C (coulomb). When describing the backdrop of tiny objects such as atoms, we utilize appropriately small units of measure out, such as the atomic mass unit of measurement (amu) and the key unit of measurement of accuse (due east). The amu was originally divers based on hydrogen, the lightest element, and so later in terms of oxygen. Since 1961, it has been defined with regard to the most arable isotope of carbon, atoms of which are assigned masses of exactly 12 amu. (This isotope is known as "carbon-12" every bit will exist discussed later in this module.) Thus, one amu is exactly [latex]\frac{1}{12}[/latex] of the mass of one carbon-12 atom: 1 amu = 1.6605 × 10−24 one thousand. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of measurement of charge (also called the simple charge) equals the magnitude of the charge of an electron (eastward) with eastward = 1.602 × 10−nineteen C.
A proton has a mass of 1.0073 amu and a charge of i+. A neutron is a slightly heavier particle with a mass ane.0087 amu and a charge of zero; every bit its proper noun suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of almost 0.00055 amu (it would take almost 1800 electrons to equal the mass of ane proton. The properties of these primal particles are summarized in Tabular array iii. (An observant student might notice that the sum of an atom's subatomic particles does not equal the atom'south actual mass: The total mass of six protons, six neutrons, and six electrons is 12.0993 amu, slightly larger than 12.00 amu. This "missing" mass is known as the mass defect, and y'all will learn about it in the chapter on nuclear chemistry.)
| Name | Location | Charge (C) | Unit Charge | Mass (amu) | Mass (one thousand) |
|---|---|---|---|---|---|
| electron | outside nucleus | −i.602 × 10−19 | 1− | 0.00055 | 0.00091 × ten−24 |
| proton | nucleus | i.602 × ten−nineteen | 1+ | ane.00727 | 1.67262 × 10−24 |
| neutron | nucleus | 0 | 0 | 1.00866 | 1.67493 × 10−24 |
| Table iii. Properties of Subatomic Particles | |||||
The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element: Its value determines the identity of the atom. For case, any atom that contains 6 protons is the element carbon and has the atomic number half dozen, regardless of how many neutrons or electrons it may accept. A neutral atom must contain the aforementioned number of positive and negative charges, and then the number of protons equals the number of electrons. Therefore, the diminutive number also indicates the number of electrons in an atom. The total number of protons and neutrons in an atom is chosen its mass number (A). The number of neutrons is therefore the deviation between the mass number and the atomic number: A – Z = number of neutrons.
[latex]\brainstorm{array}{r @ {{}={}} l} \text{atomic number (Z)} & \text{number of protons} \\[1em] \text{mass number (A)} & \text{number of protons + number of neutrons} \\[1em] \text{A - Z} & \text{number of neutrons} \end{array}[/latex]
Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. The charge of an atom is divers as follows:
Atomic accuse = number of protons − number of electrons
Equally will be discussed in more detail later on in this chapter, atoms (and molecules) typically larn charge by gaining or losing electrons. An atom that gains one or more electrons volition showroom a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (Z = xi) has 11 electrons. If this atom loses one electron, it will go a cation with a one+ accuse (11 − 10 = ane+). A neutral oxygen cantlet (Z = 8) has eight electrons, and if it gains two electrons it will become an anion with a 2− charge (8 − 10 = 2−).
Example 1
Composition of an Atom
Iodine is an essential trace element in our nutrition; it is needed to produce thyroid hormone. Insufficient iodine in the diet can pb to the development of a goiter, an enlargement of the thyroid gland (Figure ii).
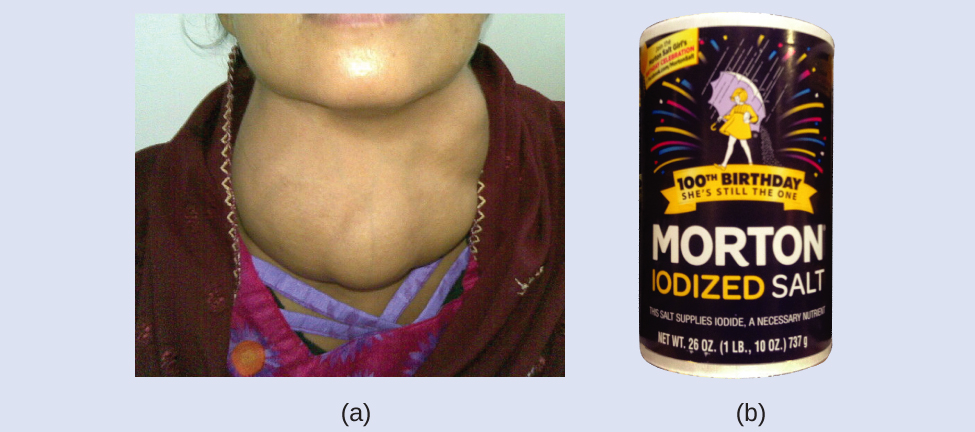
The addition of small amounts of iodine to table salt (iodized salt) has essentially eliminated this health concern in the United States, but every bit much as xl% of the world's population is all the same at gamble of iodine deficiency. The iodine atoms are added as anions, and each has a 1− charge and a mass number of 127. Decide the numbers of protons, neutrons, and electrons in one of these iodine anions.
Solution
The atomic number of iodine (53) tells us that a neutral iodine cantlet contains 53 protons in its nucleus and 53 electrons outside its nucleus. Considering the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 – (ane–) = 54].
Check Your Learning
An ion of platinum has a mass number of 195 and contains 74 electrons. How many protons and neutrons does it comprise, and what is its charge?
Answer:
78 protons; 117 neutrons; charge is 4+
Chemical Symbols
A chemic symbol is an abbreviation that nosotros employ to betoken an element or an cantlet of an element. For instance, the symbol for mercury is Hg (Effigy 3). We utilize the aforementioned symbol to indicate ane atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).

The symbols for several common elements and their atoms are listed in Table 4. Some symbols are derived from the common name of the element; others are abbreviations of the name in another language. Nearly symbols take one or 2 letters, but three-letter symbols take been used to draw some elements that have diminutive numbers greater than 112. To avert confusion with other notations, just the get-go letter of a symbol is capitalized. For instance, Co is the symbol for the element cobalt, but CO is the notation for the compound carbon monoxide, which contains atoms of the elements carbon (C) and oxygen (O). All known elements and their symbols are in the periodic table in Effigy 2 in Chapter 2.v The Periodic Table (also found in Appendix A).
| Element | Symbol | Chemical element | Symbol |
|---|---|---|---|
| aluminum | Al | fe | Fe (from ferrum) |
| bromine | Br | lead | Atomic number 82 (from plumbum) |
| calcium | Ca | magnesium | Mg |
| carbon | C | mercury | Hg (from hydrargyrum) |
| chlorine | Cl | nitrogen | N |
| chromium | Cr | oxygen | O |
| cobalt | Co | potassium | G (from kalium) |
| copper | Cu (from cuprum) | silicon | Si |
| fluorine | F | silver | Ag (from argentum) |
| gold | Au (from aurum) | sodium | Na (from natrium) |
| helium | He | sulfur | South |
| hydrogen | H | tin | Sn (from stannum) |
| iodine | I | zinc | Zn |
| Table iv. Some Common Elements and Their Symbols | |||
Traditionally, the discoverer (or discoverers) of a new element names the element. Nevertheless, until the proper name is recognized by the International Spousal relationship of Pure and Applied Chemical science (IUPAC), the recommended name of the new element is based on the Latin word(southward) for its diminutive number. For example, chemical element 106 was chosen unnilhexium (Unh), chemical element 107 was called unnilseptium (Uns), and element 108 was called unniloctium (Uno) for several years. These elements are now named later on scientists (or occasionally locations); for instance, element 106 is now known equally seaborgium (Sg) in honor of Glenn Seaborg, a Nobel Prize winner who was active in the discovery of several heavy elements.

Visit this site to learn more about IUPAC, the International Union of Pure and Applied Chemical science, and explore its periodic table.
Isotopes
The symbol for a specific isotope of any element is written past placing the mass number equally a superscript to the left of the element symbol (Figure 4). The atomic number is sometimes written as a subscript preceding the symbol, merely since this number defines the element's identity, as does its symbol, it is frequently omitted. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can exist identified every bit 24Mg, 25Mg, and 26Mg. These isotope symbols are read every bit "element, mass number" and can be symbolized consequent with this reading. For case, 24Mg is read every bit "magnesium 24," and can be written every bit "magnesium-24" or "Mg-24." 25Mg is read as "magnesium 25," and can be written as "magnesium-25" or "Mg-25." All magnesium atoms take 12 protons in their nucleus. They differ just considering a 24Mg cantlet has 12 neutrons in its nucleus, a 25Mg cantlet has 13 neutrons, and a 26Mg has 14 neutrons.
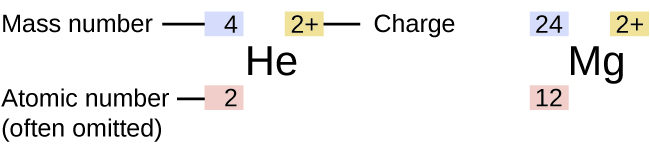
Information about the naturally occurring isotopes of elements with diminutive numbers 1 through 10 is given in Tabular array 5. Notation that in addition to standard names and symbols, the isotopes of hydrogen are frequently referred to using common names and accompanying symbols. Hydrogen-2, symbolized twoH, is also chosen deuterium and sometimes symbolized D. Hydrogen-iii, symbolized iiiH, is as well called tritium and sometimes symbolized T.
| Chemical element | Symbol | Atomic Number | Number of Protons | Number of Neutrons | Mass (amu) | % Natural Abundance |
|---|---|---|---|---|---|---|
| hydrogen | [latex]_1^1\text{H}[/latex] (protium) | 1 | 1 | 0 | 1.0078 | 99.989 |
| [latex]_1^two\text{H}[/latex] (deuterium) | 1 | i | one | 2.0141 | 0.0115 | |
| [latex]_1^3\text{H}[/latex] (tritium) | i | 1 | 2 | 3.01605 | — (trace) | |
| helium | [latex]_2^3\text{He}[/latex] | 2 | 2 | 1 | 3.01603 | 0.00013 |
| [latex]_2^4\text{He}[/latex] | two | 2 | 2 | 4.0026 | 100 | |
| lithium | [latex]_3^6\text{Li}[/latex] | 3 | 3 | 3 | half dozen.0151 | 7.59 |
| [latex]_3^7\text{Li}[/latex] | 3 | iii | 4 | 7.0160 | 92.41 | |
| glucinium | [latex]_4^9\text{Be}[/latex] | four | 4 | five | nine.0122 | 100 |
| boron | [latex]_5^{10}\text{B}[/latex] | 5 | 5 | v | 10.0129 | 19.nine |
| [latex]_5^{eleven}\text{B}[/latex] | 5 | v | 6 | eleven.0093 | 80.i | |
| carbon | [latex]_6^{12}\text{C}[/latex] | six | 6 | six | 12.0000 | 98.89 |
| [latex]_6^{13}\text{C}[/latex] | 6 | 6 | vii | xiii.0034 | one.11 | |
| [latex]_6^{14}\text{C}[/latex] | six | 6 | 8 | xiv.0032 | — (trace) | |
| nitrogen | [latex]_7^{14}\text{N}[/latex] | 7 | seven | seven | xiv.0031 | 99.63 |
| [latex]_7^{15}\text{Due north}[/latex] | seven | 7 | eight | xv.0001 | 0.37 | |
| oxygen | [latex]_8^{16}\text{O}[/latex] | 8 | eight | 8 | 15.9949 | 99.757 |
| [latex]_8^{17}\text{O}[/latex] | 8 | 8 | 9 | 16.9991 | 0.038 | |
| [latex]_8^{18}\text{O}[/latex] | 8 | 8 | 10 | 17.9992 | 0.205 | |
| fluorine | [latex]_9^{19}\text{F}[/latex] | 9 | 9 | 10 | eighteen.9984 | 100 |
| neon | [latex]_{ten}^{20}\text{Ne}[/latex] | ten | 10 | x | 19.9924 | ninety.48 |
| [latex]_{x}^{21}\text{Ne}[/latex] | ten | 10 | eleven | 20.9938 | 0.27 | |
| [latex]_{10}^{22}\text{Ne}[/latex] | ten | ten | 12 | 21.9914 | ix.25 | |
| Tabular array 5.Nuclear Compositions of Atoms of the Very Calorie-free Elements | ||||||

Use this Build an Cantlet simulator to build atoms of the first ten elements, meet which isotopes exist, check nuclear stability, and proceeds experience with isotope symbols.
Diminutive Mass
Because each proton and each neutron contribute approximately one amu to the mass of an cantlet, and each electron contributes far less, the atomic mass of a single atom is approximately equal to its mass number (a whole number). Nevertheless, the boilerplate masses of atoms of near elements are not whole numbers because virtually elements exist naturally as mixtures of two or more isotopes.
The mass of an element shown in a periodic tabular array or listed in a tabular array of atomic masses is a weighted, average mass of all the isotopes present in a naturally occurring sample of that element. This is equal to the sum of each individual isotope's mass multiplied by its fractional abundance.
[latex]\displaystyle{} \text{boilerplate mass} = \sum_{i} (\text{partial abundance} \times \text{isotopic mass})_{i}[/latex]
For instance, the element boron is composed of two isotopes: About nineteen.9% of all boron atoms are xB with a mass of 10.0129 amu, and the remaining 80.1% are 11B with a mass of eleven.0093 amu. The average atomic mass for boron is calculated to be:
[latex]\begin{assortment}{r @{{}={}} l} \text{boron average mass} & (0.199 \times x.0129 \;\text{amu}) + (0.801 \times 11.0093 \;\text{amu}) \\[1em] & 1.99 \;\text{amu} + viii.82 \;\text{amu} \\[1em] & ten.81 \;\text{amu} \cease{array}[/latex]
It is important to understand that no single boron atom weighs exactly ten.8 amu; ten.viii amu is the average mass of all boron atoms, and individual boron atoms weigh either approximately 10 amu or 11 amu.
Instance two
Calculation of Average Atomic Mass
A meteorite found in key Indiana contains traces of the noble gas neon picked upwards from the solar wind during the meteorite'southward trip through the solar system. Analysis of a sample of the gas showed that it consisted of 91.84% 20Ne (mass 19.9924 amu), 0.47% 21Ne (mass 20.9940 amu), and 7.69% 22Ne (mass 21.9914 amu). What is the average mass of the neon in the solar current of air?
Solution
[latex]\brainstorm{array}{r @{{}={}} l} \text{average mass} & (0.9184 \times 19.9924 \;\text{amu}) + (0.0047 \times twenty.9940 \;\text{amu})+(0.0769 \times 21.9914 \;\text{amu}) \\[1em] & (18.36+0.099+1.69) \;\text{amu} \\[1em] & 20.15 \;\text{amu} \end{array}[/latex]
The average mass of a neon atom in the solar wind is xx.15 amu. (The boilerplate mass of a terrestrial neon atom is 20.1796 amu. This result demonstrates that we may find slight differences in the natural abundance of isotopes, depending on their origin.)
Check Your Learning
A sample of magnesium is found to contain 78.lxx% of 24Mg atoms (mass 23.98 amu), 10.13% of 25Mg atoms (mass 24.99 amu), and 11.17% of 26Mg atoms (mass 25.98 amu). Summate the average mass of a Mg cantlet.
We tin also exercise variations of this type of calculation, every bit shown in the next example.
Example three
Calculation of Percent Abundance
Naturally occurring chlorine consists of 35Cl (mass 34.96885 amu) and 37Cl (mass 36.96590 amu), with an boilerplate mass of 35.453 amu. What is the per centum limerick of Cl in terms of these two isotopes?
Solution
The average mass of chlorine is the fraction that is 35Cl times the mass of 35Cl plus the fraction that is 37Cl times the mass of 37Cl.
[latex]\text{average mass} = (\text{fraction of} \ ^{35}\text{Cl} \ \times \ \text{mass of} \ ^{35}\text{Cl}) + (\text{fraction of} \ ^{37}\text{Cl} \ \times \ \text{mass of} \ ^{37}\text{Cl})[/latex]
If we let x represent the fraction that is 35Cl, then the fraction that is 37Cl is represented by one.00 − x.
(The fraction that is 35Cl + the fraction that is 37Cl must add up to 1, and so the fraction of 37Cl must equal 1.00 − the fraction of 35Cl.)
Substituting this into the boilerplate mass equation, we accept:
[latex]\brainstorm{array}{r @{{}={}} 50}35.453 \;\text{amu} & (ten \times 34.96885 \;\text{amu}) + [(1.00 - x) \times 36.96590\;\text{amu}] \\[1em] 35.453 & 34.96885x + 36.96590 - 36.96590x \\[1em] ane.99705x & 1.513 \\[1em] x & \frac{i.513}{1.99705} = 0.7576 \terminate{array}[/latex]
So solving yields: x = 0.7576, which means that i.00 − 0.7576 = 0.2424. Therefore, chlorine consists of 75.76% 35Cl and 24.24% 37Cl.
Check Your Learning
Naturally occurring copper consists of 63Cu (mass 62.9296 amu) and 65Cu (mass 64.9278 amu), with an average mass of 63.546 amu. What is the percentage composition of Cu in terms of these two isotopes?
Answer:
69.15% Cu-63 and 30.85% Cu-65

Visit this site to brand mixtures of the master isotopes of the commencement 18 elements, proceeds experience with average atomic mass, and check naturally occurring isotope ratios using the Isotopes and Atomic Mass simulation.
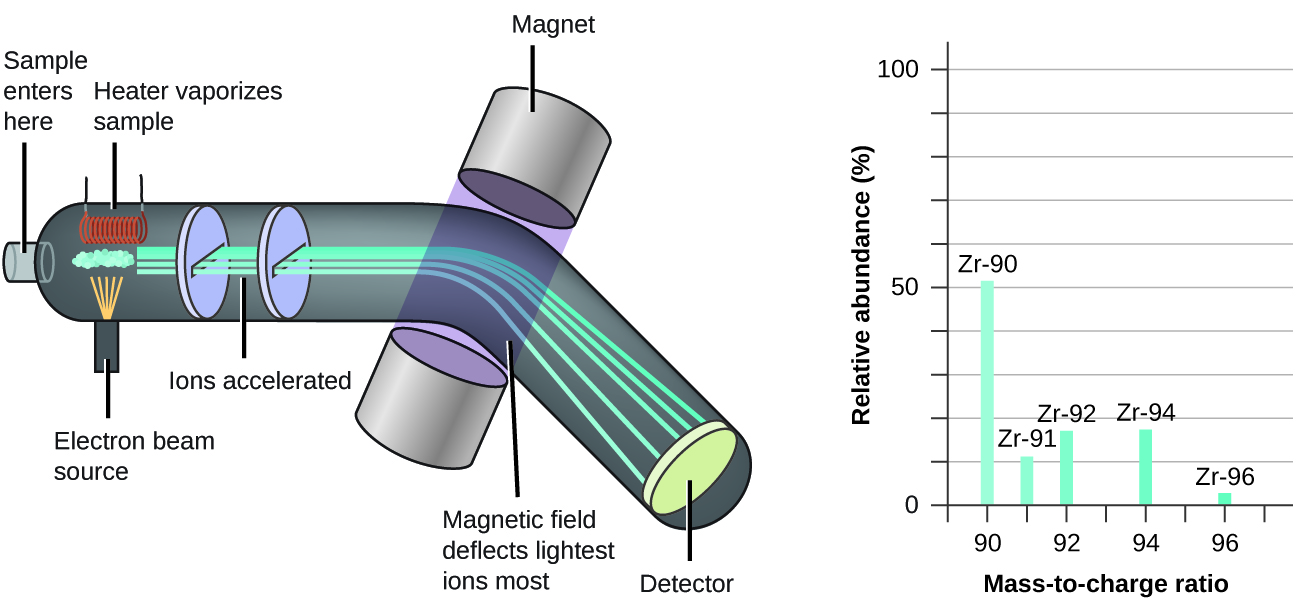
The occurrence and natural abundances of isotopes tin can be experimentally determined using an musical instrument called a mass spectrometer. Mass spectrometry (MS) is widely used in chemistry, forensics, medicine, environmental scientific discipline, and many other fields to analyze and assist place the substances in a sample of cloth. In a typical mass spectrometer (Figure five), the sample is vaporized and exposed to a high-energy electron axle that causes the sample's atoms (or molecules) to become electrically charged, typically by losing one or more electrons. These cations then pass through a (variable) electric or magnetic field that deflects each cation'southward path to an extent that depends on both its mass and accuse (similar to how the path of a large steel ball begetting rolling past a magnet is deflected to a lesser extent that that of a minor steel BB). The ions are detected, and a plot of the relative number of ions generated versus their mass-to-charge ratios (a mass spectrum) is made. The height of each vertical feature or peak in a mass spectrum is proportional to the fraction of cations with the specified mass-to-charge ratio. Since its initial utilise during the development of mod atomic theory, MS has evolved to become a powerful tool for chemical analysis in a wide range of applications.

Come across an animation that explains mass spectrometry. Watch this video from the Regal Society for Chemistry for a brief description of the rudiments of mass spectrometry.
Primal Concepts and Summary
An atom consists of a pocket-size, positively charged nucleus surrounded by electrons. The nucleus contains protons and neutrons; its diameter is most 100,000 times smaller than that of the cantlet. The mass of ane atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is divers as exactly [latex]\frac{1}{12}[/latex] of the mass of a carbon-12 atom and is equal to 1.6605 × 10−24 g.
Protons are relatively heavy particles with a charge of ane+ and a mass of i.0073 amu. Neutrons are relatively heavy particles with no accuse and a mass of i.0087 amu. Electrons are light particles with a charge of 1− and a mass of 0.00055 amu. The number of protons in the nucleus is called the atomic number (Z) and is the belongings that defines an atom's elemental identity. The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. An atom is neutral when it contains equal numbers of electrons and protons.
Isotopes of an element are atoms with the same atomic number simply different mass numbers; isotopes of an element, therefore, differ from each other but in the number of neutrons within the nucleus. When a naturally occurring element is composed of several isotopes, the diminutive mass of the element represents the boilerplate of the masses of the isotopes involved. A chemical symbol identifies the atoms in a substance using symbols, which are one-, two-, or three-letter abbreviations for the atoms.
Key Equations
- [latex]\displaystyle{} \text{average mass} = \sum_{i} (\text{partial abundance} \times \text{isotopic mass})_i[/latex]
Chemistry End of Chapter Exercises
- In what manner are isotopes of a given chemical element e'er different? In what fashion(s) are they ever the same?
- Write the symbol for each of the following ions:
(a) the ion with a ane+ charge, atomic number 55, and mass number 133
(b) the ion with 54 electrons, 53 protons, and 74 neutrons
(c) the ion with diminutive number 15, mass number 31, and a 3− charge
(d) the ion with 24 electrons, 30 neutrons, and a three+ charge
- Write the symbol for each of the post-obit ions:
(a) the ion with a 3+ charge, 28 electrons, and a mass number of 71
(b) the ion with 36 electrons, 35 protons, and 45 neutrons
(c) the ion with 86 electrons, 142 neutrons, and a 4+ charge
(d) the ion with a 2+ charge, diminutive number 38, and mass number 87
- Open the Build an Atom simulation and click on the Atom icon.
(a) Pick whatsoever one of the outset ten elements that you would like to build and state its symbol.
(b) Drag protons, neutrons, and electrons onto the atom template to make an atom of your chemical element.
Country the numbers of protons, neutrons, and electrons in your atom, as well as the net accuse and mass number.
(c) Click on "Net Charge" and "Mass Number," bank check your answers to (b), and right, if needed.
(d) Predict whether your atom volition be stable or unstable. Land your reasoning.
(eastward) Check the "Stable/Unstable" box. Was your answer to (d) correct? If not, first predict what you can do to brand a stable atom of your element, and then practise it and see if information technology works. Explain your reasoning.
- Open the Build an Cantlet simulation
(a) Drag protons, neutrons, and electrons onto the cantlet template to make a neutral cantlet of Oxygen-16 and give the isotope symbol for this atom.
(b) Now add two more than electrons to make an ion and give the symbol for the ion y'all take created.
- Open the Build an Atom simulation
(a) Elevate protons, neutrons, and electrons onto the atom template to make a neutral atom of Lithium-vi and requite the isotope symbol for this atom.
(b) Now remove one electron to make an ion and give the symbol for the ion you have created.
- Determine the number of protons, neutrons, and electrons in the post-obit isotopes that are used in medical diagnoses:
(a) atomic number ix, mass number 18, charge of ane−
(b) atomic number 43, mass number 99, accuse of vii+
(c) atomic number 53, atomic mass number 131, charge of 1−
(d) atomic number 81, atomic mass number 201, accuse of one+
(e) Name the elements in parts (a), (b), (c), and (d).
- The following are properties of isotopes of two elements that are essential in our nutrition. Decide the number of protons, neutrons and electrons in each and proper name them.
(a) atomic number 26, mass number 58, charge of 2+
(b) diminutive number 53, mass number 127, charge of 1−
- Give the number of protons, electrons, and neutrons in neutral atoms of each of the post-obit isotopes:
(a) [latex]_5^{10}\text{B}[/latex]
(b) [latex]_{80}^{199}\text{Hg}[/latex]
(c) [latex]_{29}^{63}\text{Cu}[/latex]
(d) [latex]_6^{13}\text{C}[/latex]
(e) [latex]_{34}^{77}\text{Se}[/latex]
- Requite the number of protons, electrons, and neutrons in neutral atoms of each of the following isotopes:
(a) [latex]_3^7\text{Li}[/latex]
(b) [latex]_{52}^{125}\text{Te}[/latex]
(c) [latex]_{47}^{109}\text{Ag}[/latex]
(d) [latex]_{seven}^{15}\text{Northward}[/latex]
(e) [latex]_{15}^{31}\text{P}[/latex]
- Click on the site and select the "Mix Isotopes" tab, hibernate the "Percent Composition" and "Average Atomic Mass" boxes, and and so select the element boron.
(a) Write the symbols of the isotopes of boron that are shown as naturally occurring in significant amounts.
(b) Predict the relative amounts (percentages) of these boron isotopes plant in nature. Explicate the reasoning behind your option.
(c) Add isotopes to the black box to make a mixture that matches your prediction in (b). You may drag isotopes from their bins or click on "More" and then move the sliders to the advisable amounts.
(d) Reveal the "Percent Composition" and "Average Atomic Mass" boxes. How well does your mixture friction match with your prediction? If necessary, accommodate the isotope amounts to match your prediction.
(e) Select "Nature's" mix of isotopes and compare it to your prediction. How well does your prediction compare with the naturally occurring mixture? Explain. If necessary, adjust your amounts to brand them lucifer "Nature's" amounts as closely as possible.
- Echo Chemistry End of Chapter Practice 11 using an chemical element that has three naturally occurring isotopes.
- An element has the following natural abundances and isotopic masses: 90.92% abundance with nineteen.99 amu, 0.26% abundance with 20.99 amu, and 8.82% affluence with 21.99 amu. Summate the boilerplate diminutive mass of this chemical element.
- Average atomic masses listed past IUPAC are based on a study of experimental results. Bromine has ii isotopes 79Br and 81Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average diminutive mass of bromine based on these experiments.
- Variations in average atomic mass may be observed for elements obtained from different sources. Lithium provides an instance of this. The isotopic composition of lithium from naturally occurring minerals is 7.5% viLi and 92.5% 7Li, which have masses of half dozen.01512 amu and seven.01600 amu, respectively. A commercial source of lithium, recycled from a military machine source, was iii.75% half-dozenLi (and the rest 7Li). Calculate the average atomic mass values for each of these two sources.
- The average atomic masses of some elements may vary, depending upon the sources of their ores. Naturally occurring boron consists of two isotopes with accurately known masses (10B, 10.0129 amu and elevenB, 11.0931 amu). The actual diminutive mass of boron can vary from x.807 to 10.819, depending on whether the mineral source is from Turkey or the United States. Summate the pct abundances leading to the two values of the boilerplate atomic masses of boron from these two countries.
- The eighteenO:sixteenO abundance ratio in some meteorites is greater than that used to calculate the average atomic mass of oxygen on earth. Is the average mass of an oxygen atom in these meteorites greater than, less than, or equal to that of a terrestrial oxygen atom?
Glossary
- anion
- negatively charged atom or molecule (contains more electrons than protons)
- atomic mass
- average mass of atoms of an element, expressed in amu
- atomic mass unit (amu)
- (likewise, unified atomic mass unit, u, or Dalton, Da) unit of mass equal to [latex]\frac{1}{12}[/latex] of the mass of a 12C cantlet
- atomic number (Z)
- number of protons in the nucleus of an cantlet
- cation
- positively charged atom or molecule (contains fewer electrons than protons)
- chemic symbol
- one-, 2-, or iii-letter of the alphabet abridgement used to represent an element or its atoms
- Dalton (Da)
- alternative unit equivalent to the atomic mass unit of measurement
- fundamental unit of charge
- (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = ane.602 × 10−19 C
- ion
- electrically charged cantlet or molecule (contains unequal numbers of protons and electrons)
- mass number (A)
- sum of the numbers of neutrons and protons in the nucleus of an atom
- unified diminutive mass unit (u)
- alternative unit equivalent to the atomic mass unit
Solutions
Answers to Chemical science End of Chapter Exercises
2. (a) 133Cs+; (b) 127I−; (c) 31P3−; (d) 57Co3+
4. (a) Carbon-12, 12C; (b) This atom contains six protons and half-dozen neutrons. There are six electrons in a neutral 12C cantlet. The internet accuse of such a neutral atom is zero, and the mass number is 12. (c) The preceding answers are correct. (d) The cantlet will be stable since C-12 is a stable isotope of carbon. (due east) The preceding respond is right. Other answers for this exercise are possible if a different element of isotope is chosen.
6. (a) Lithium-6 contains three protons, three neutrons, and 3 electrons. The isotope symbol is half dozenLi or [latex]_3^vi\text{Li}[/latex]. (b) viLi+ or [latex]_3^six \text{Li}^+[/latex]
viii. (a) Fe, 26 protons, 24 electrons, and 32 neutrons; (b) iodine, 53 protons, 54 electrons, and 74 neutrons
x. (a) 3 protons, 3 electrons, 4 neutrons; (b) 52 protons, 52 electrons, 73 neutrons; (c) 47 protons, 47 electrons, 62 neutrons; (d) seven protons, seven electrons, 8 neutrons; (eastward) 15 protons, 15 electrons, 16 neutrons
12. Let us use neon as an case. Since there are three isotopes, at that place is no way to be sure to accurately predict the abundances to make the total of 20.18 amu boilerplate atomic mass. Let us estimate that the abundances are nine% Ne-22, 91% Ne-twenty, and only a trace of Ne-21. The average mass would be 20.18 amu. Checking the nature'southward mix of isotopes shows that the abundances are 90.48% Ne-20, 9.25% Ne-22, and 0.27% Ne-21, so our guessed amounts have to be slightly adjusted.
14. 79.904 amu
16. Turkey source: 0.2649 (of 10.0129 amu isotope); The states source: 0.2537 (of ten.0129 amu isotope)
Source: https://opentextbc.ca/chemistry/chapter/2-3-atomic-structure-and-symbolism/
Posted by: prevostnotheires.blogspot.com


0 Response to "How To Find Atomic Weight Of An Element"
Post a Comment